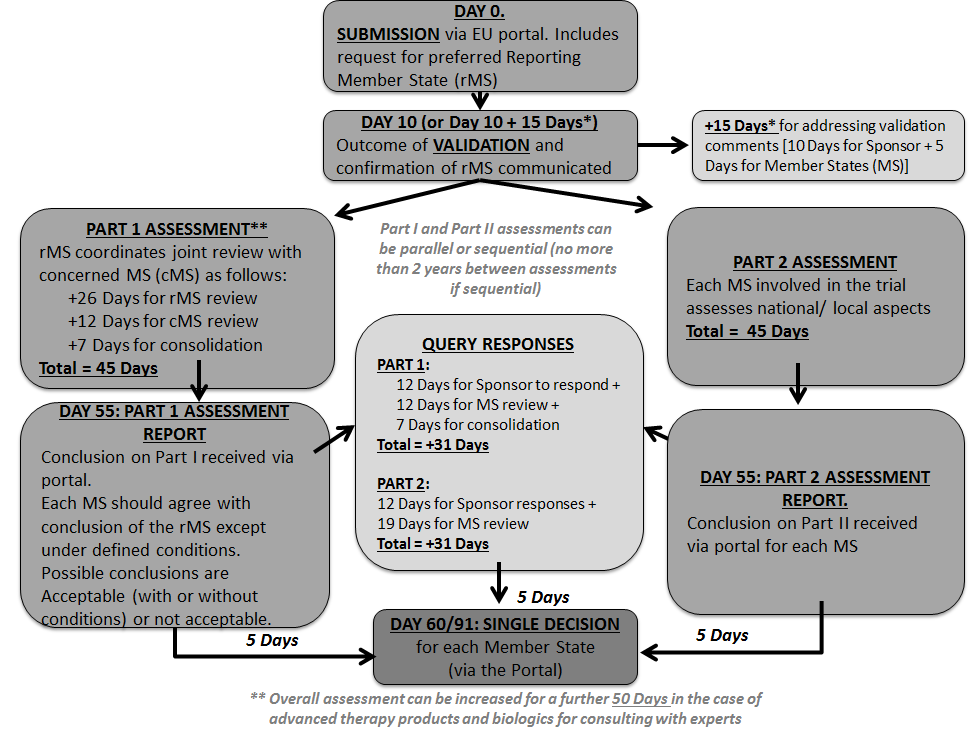
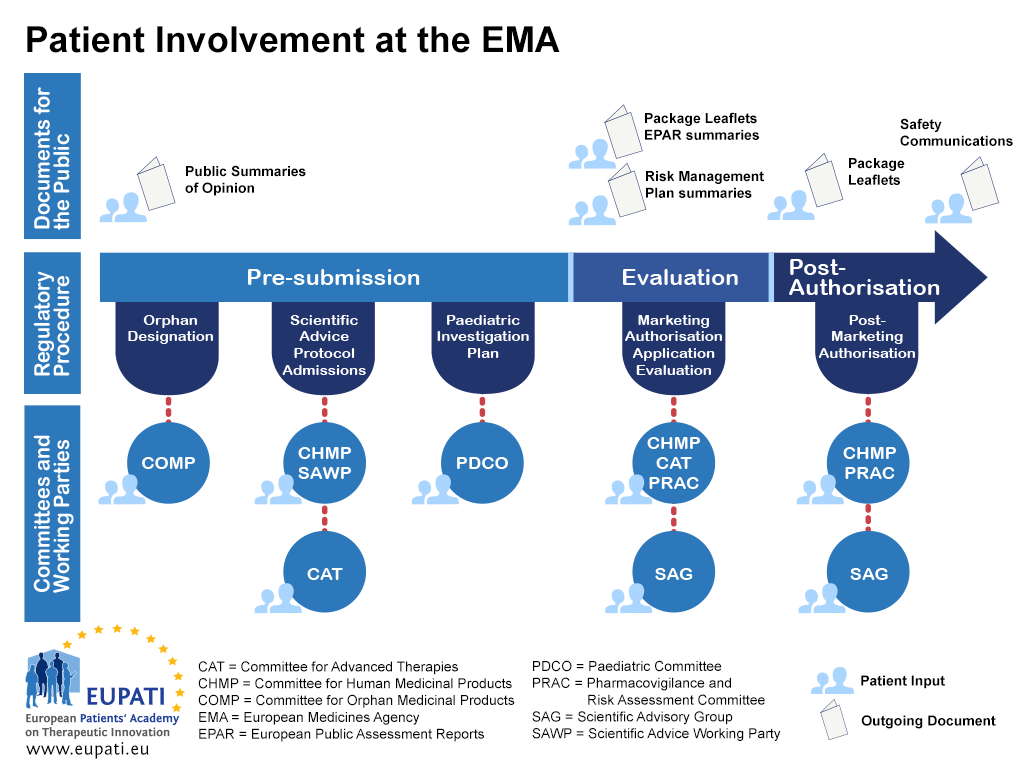
PPT – What are the EMA Guidelines for Clinical Trial Management? PowerPoint presentation | free to download - id: 91ca65-YTdlM

Regulatory compliance: How to shape a non-clinical development program and paediatric requirements - YouTube
Guideline on the requirements to the chemical and pharmaceutical quality documentation concerning investigational medicinal prod

When innovation outpaces regulations: The legal challenges for direct‐to‐patient supply of investigational medicinal products - Malone - 2022 - British Journal of Clinical Pharmacology - Wiley Online Library
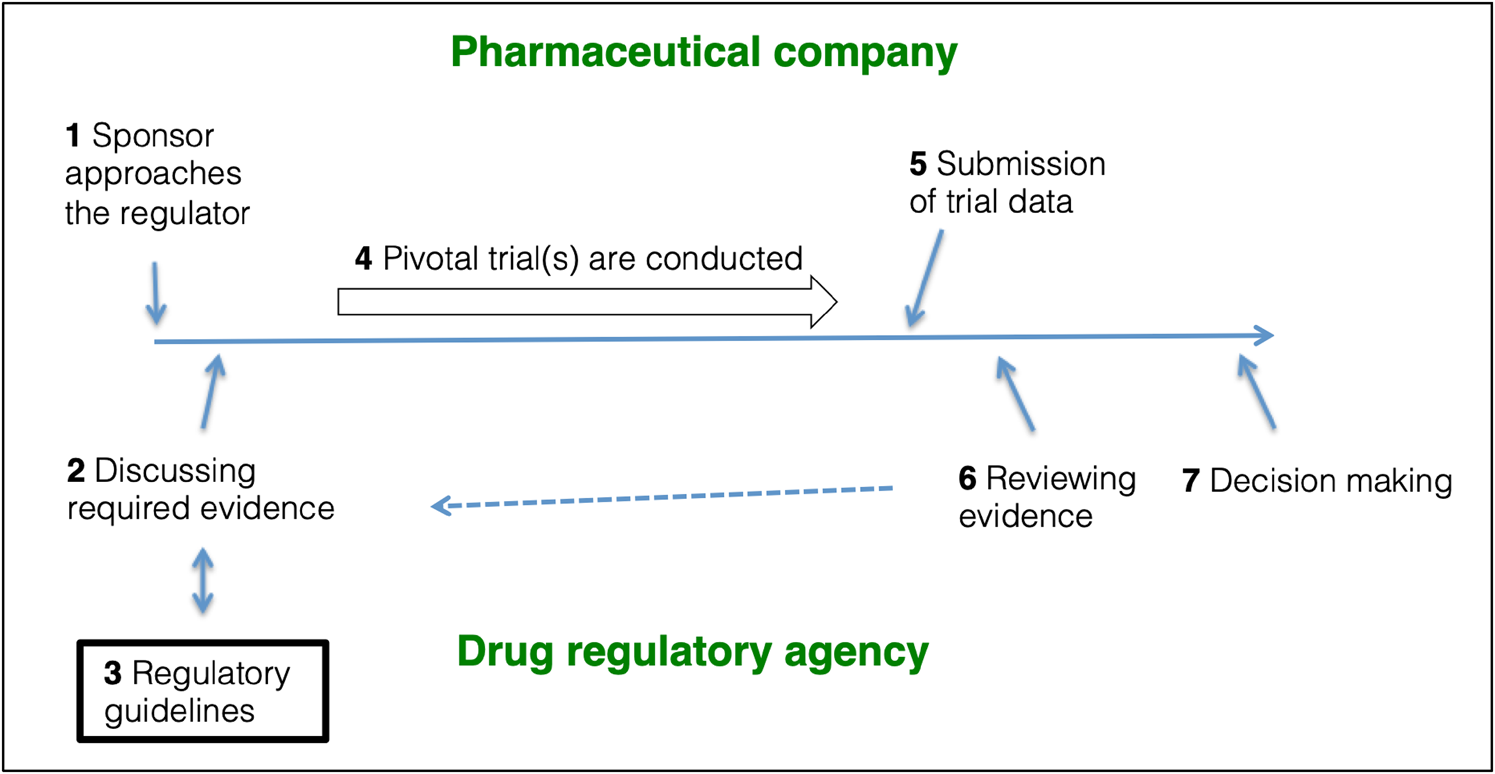
EMA and FDA psychiatric drug trial guidelines: assessment of guideline development and trial design recommendations | Epidemiology and Psychiatric Sciences | Cambridge Core
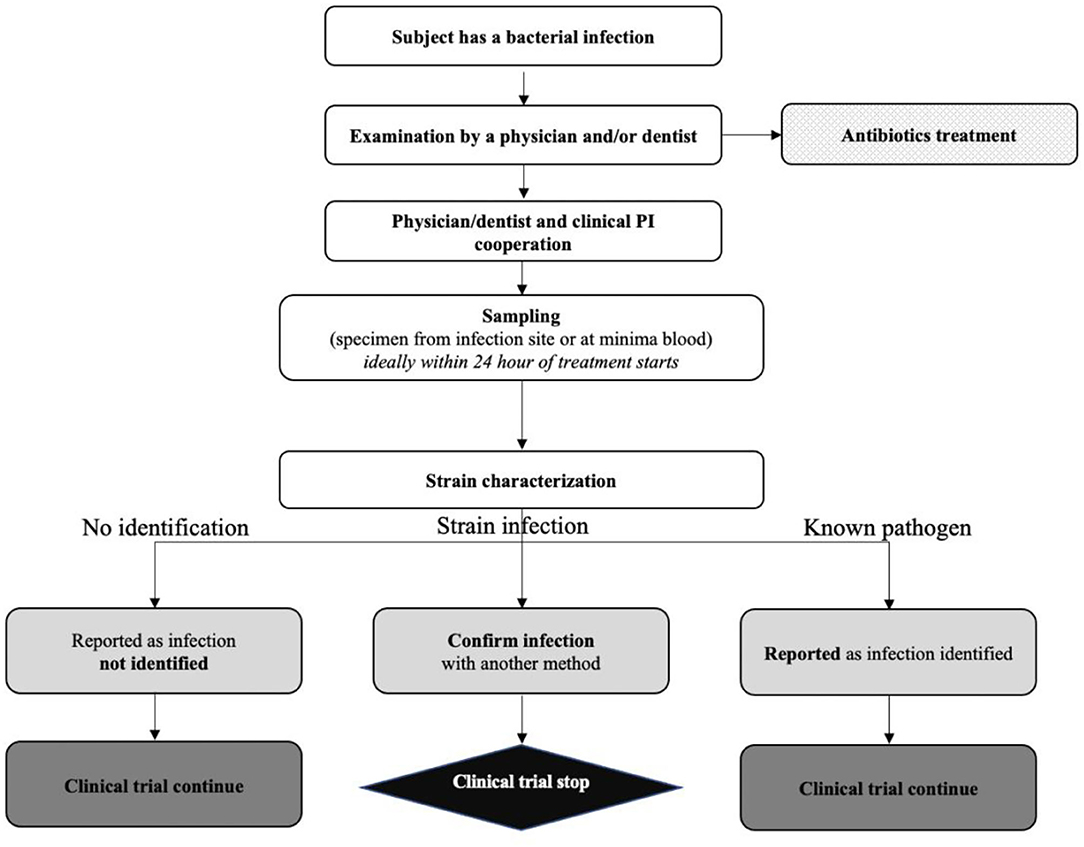
Frontiers | Entering First-in-Human Clinical Study With a Single-Strain Live Biotherapeutic Product: Input and Feedback Gained From the EMA and the FDA

EMA & FDA Approvals and Recommendations in 2020 for Oncology Drugs and Diagnostics/Devices | CATO SMS

Comparison of power calculated as per the EMA guidelines among clinical... | Download Scientific Diagram