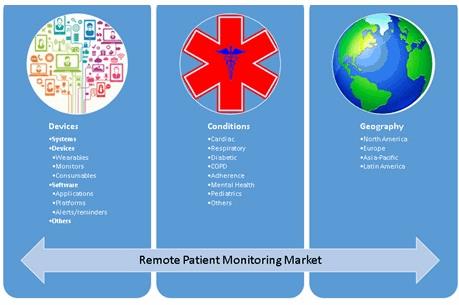
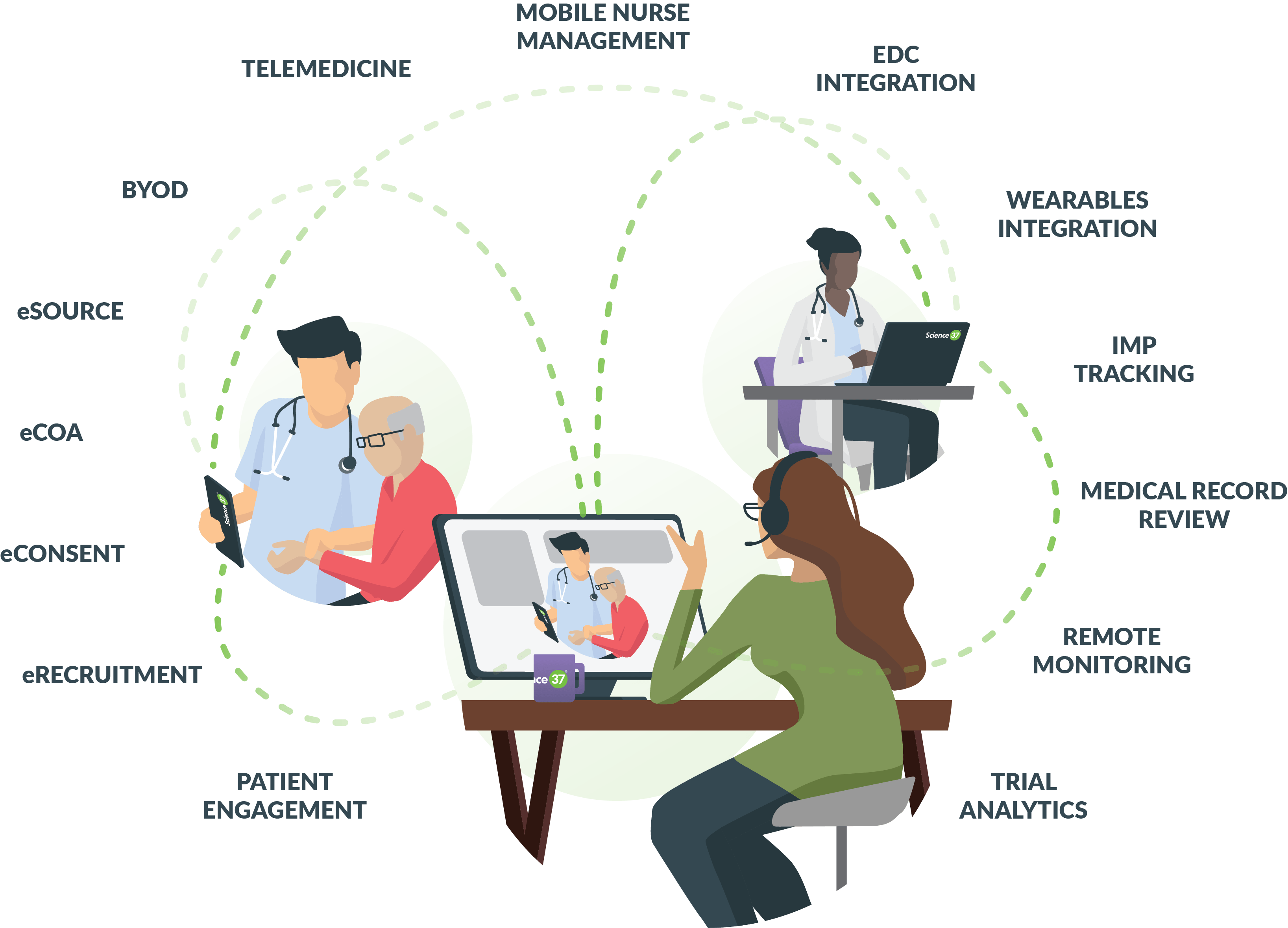
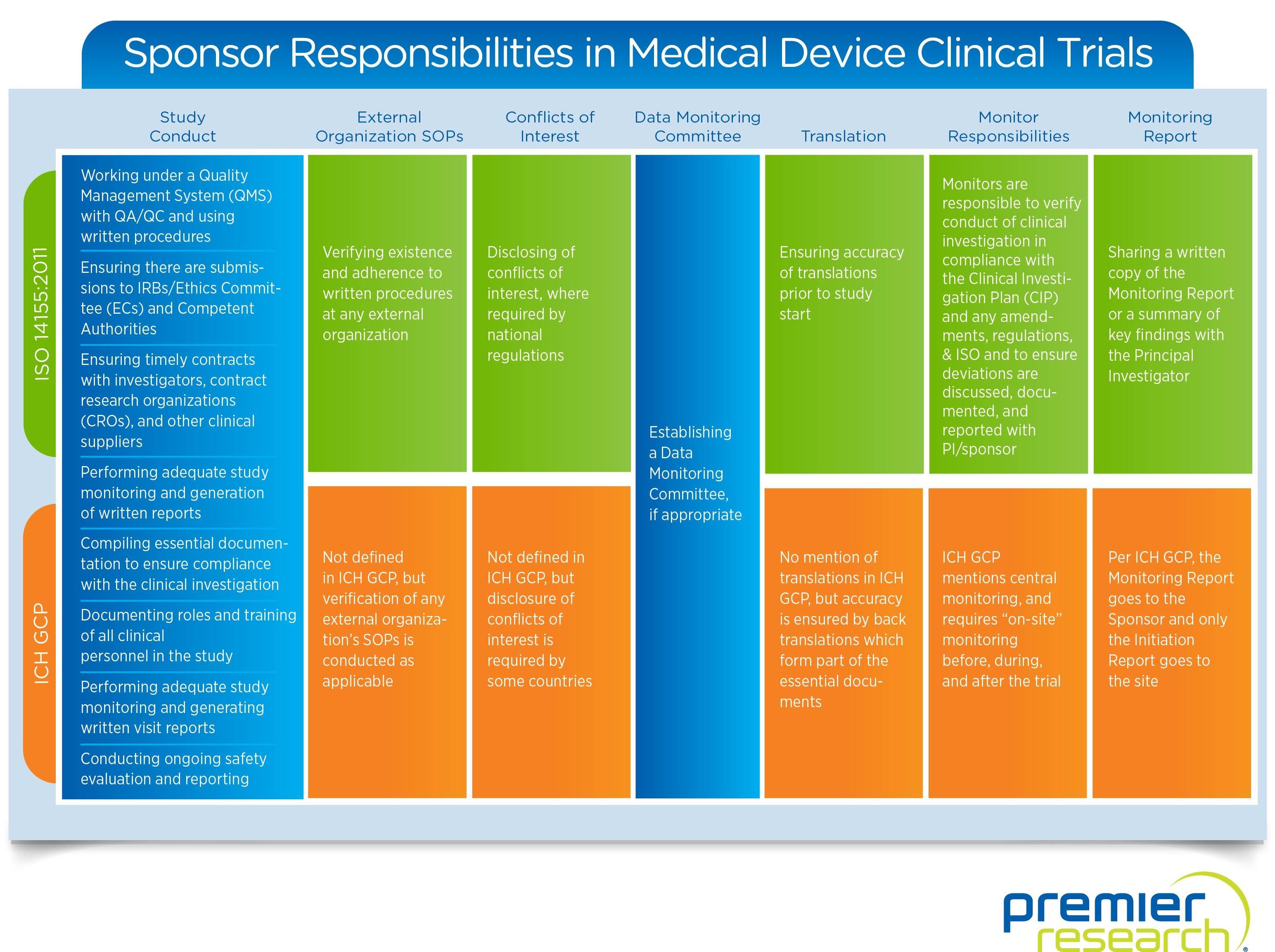
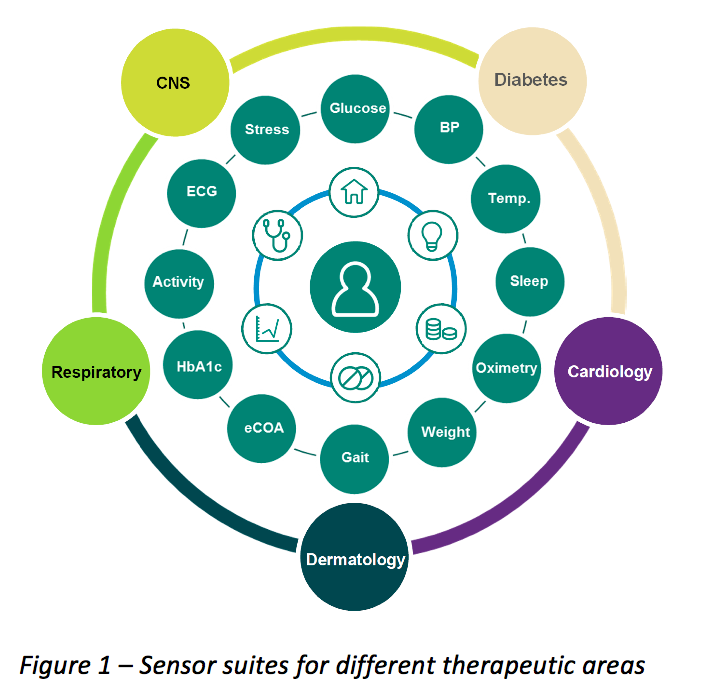
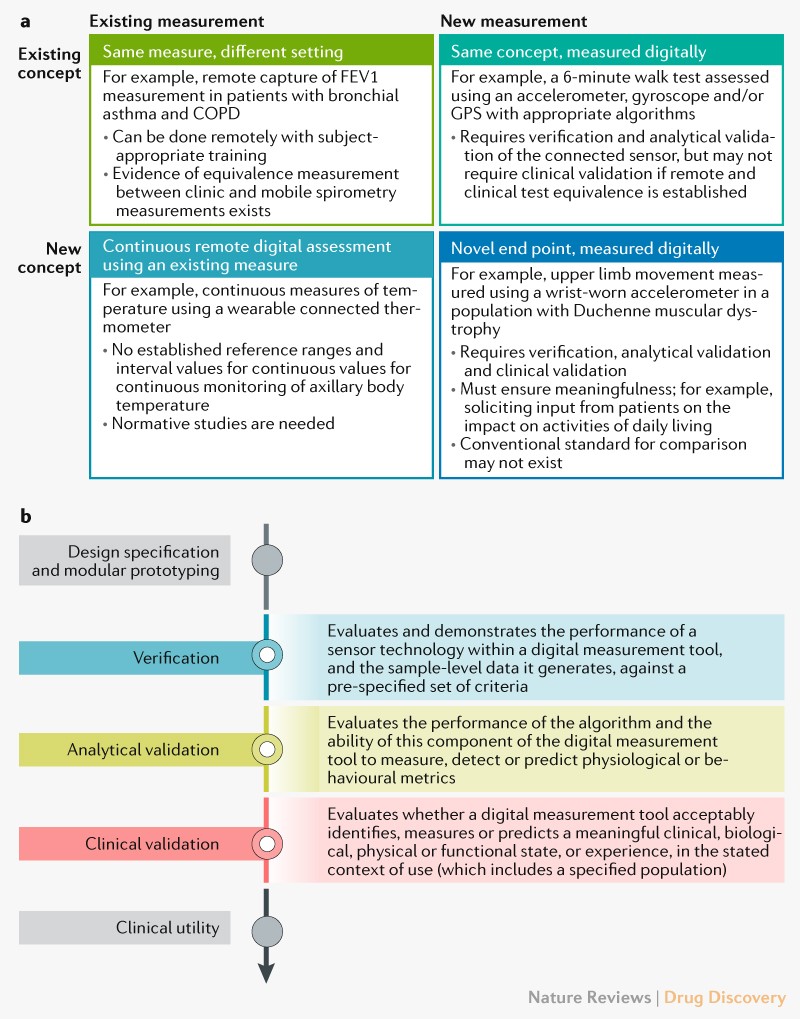
Medical Monitoring in Clinical Research - Non Clinical Physician Jobs — Clinical Research Certification
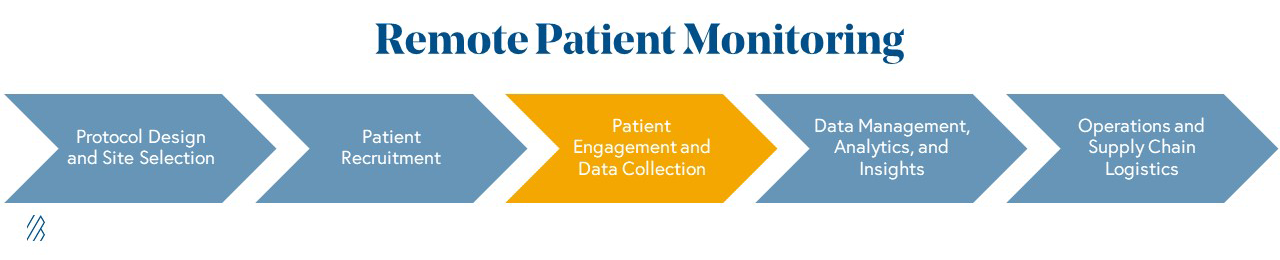
Drugs in a Virtual World: The rise of digital health solutions in clinical trials · Bessemer Venture Partners
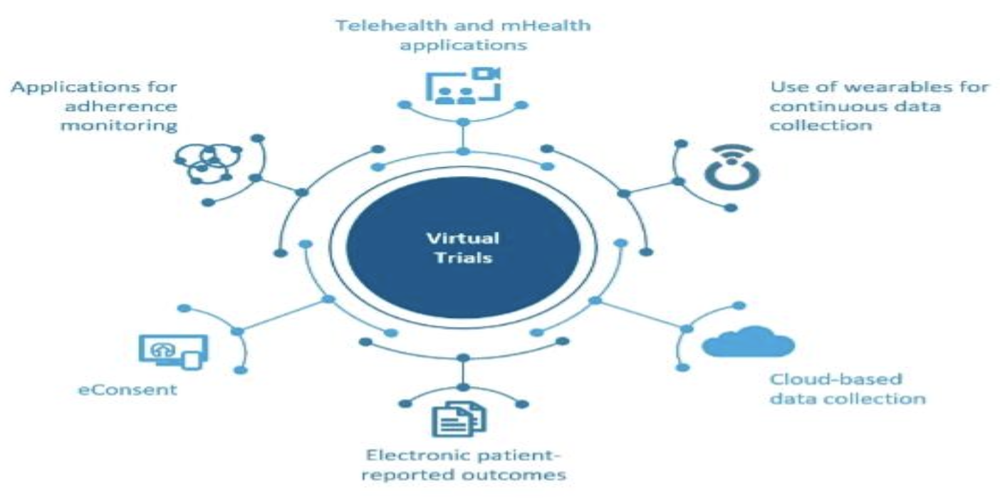
virtual clinical trials — Clinical Research Blog | Certified Clinical Research Professionals Society - Clinical Research Certification
Medical Monitoring in Clinical Research - Non Clinical Physician Jobs — Clinical Research Certification
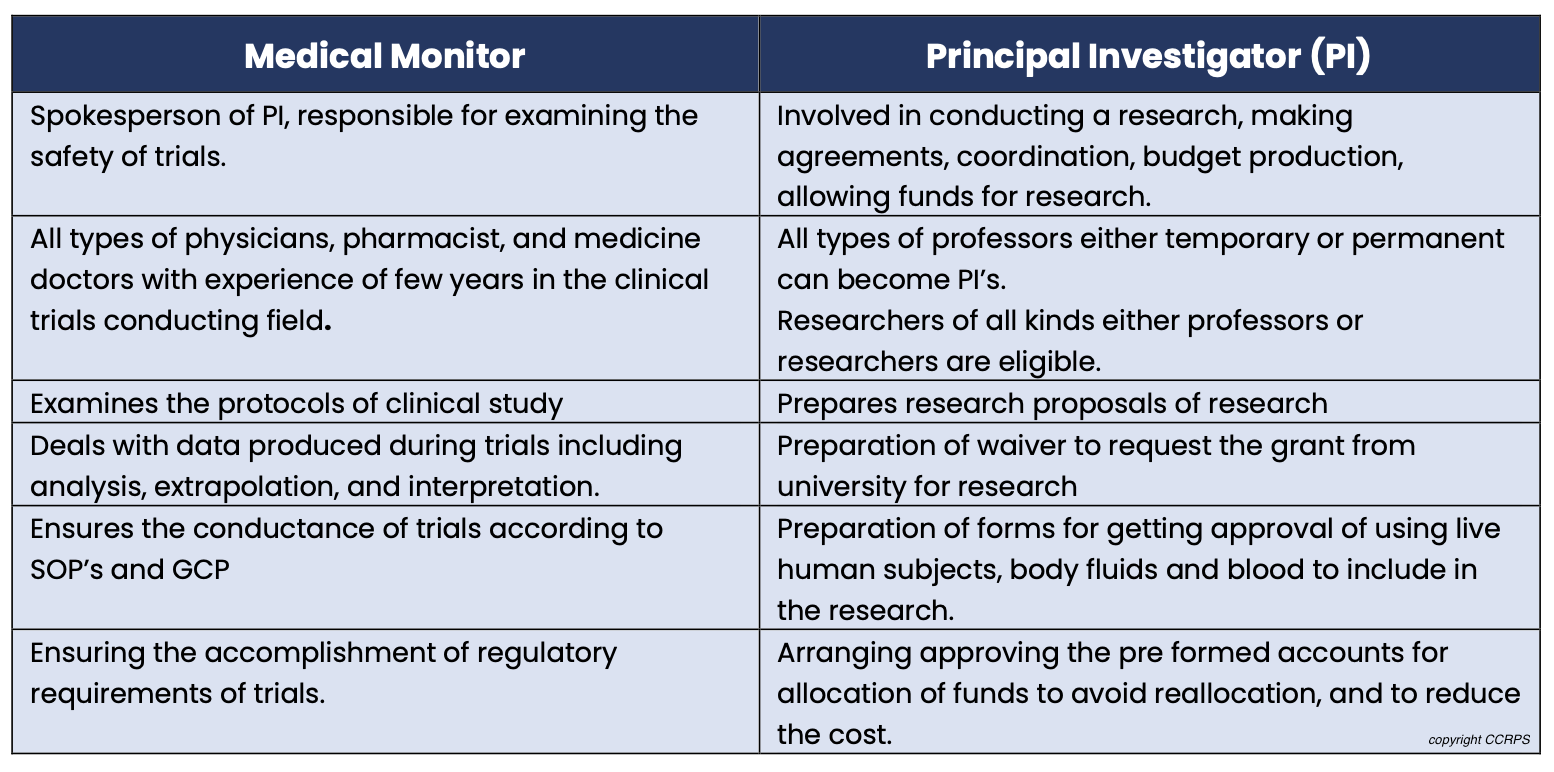
Medical Monitoring in Clinical Research - Non Clinical Physician Jobs — Clinical Research Certification
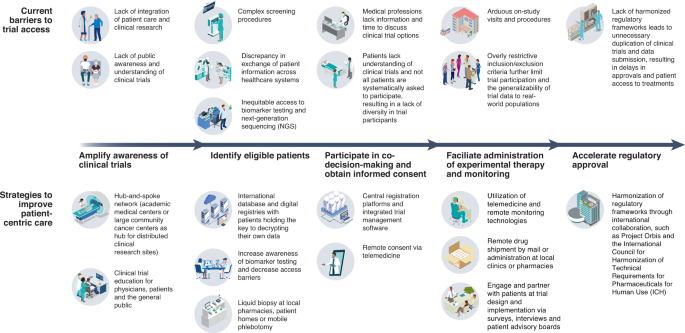
Reimagining patient-centric cancer clinical trials: a multi-stakeholder international coalition | Nature Medicine