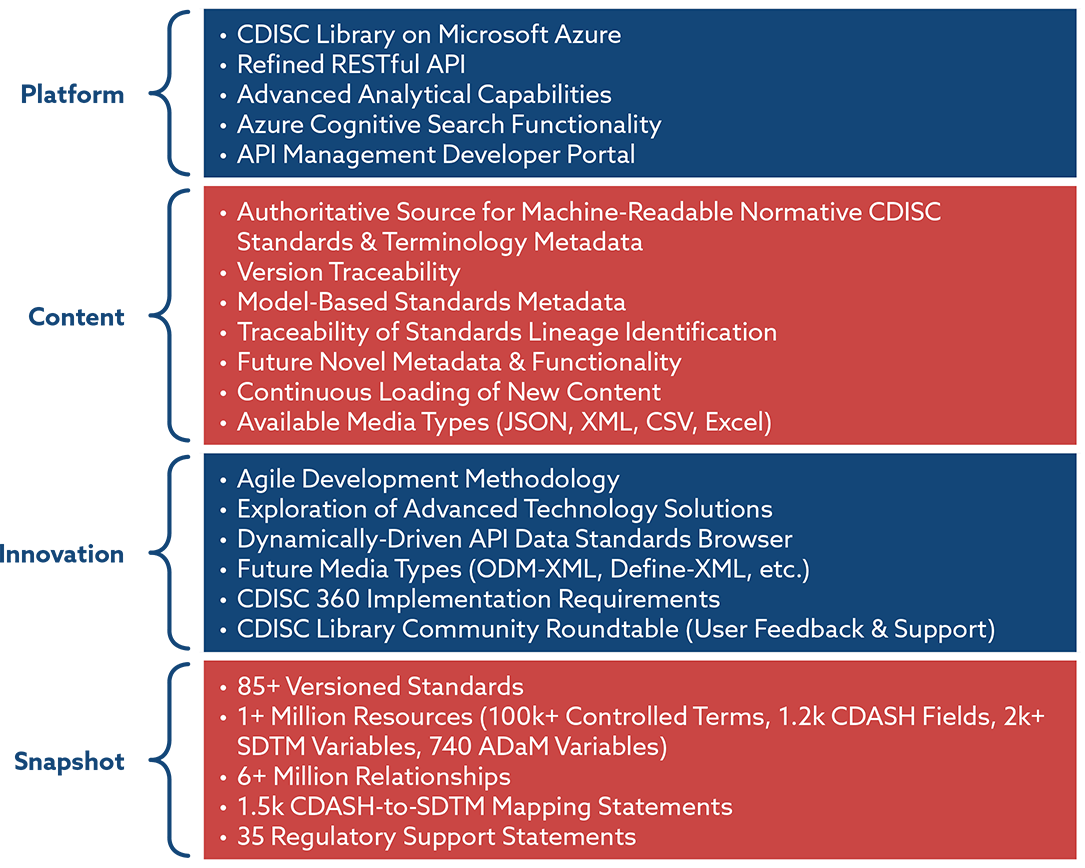
Standardizing data exchange for clinical research protocols and case report forms: An assessment of the suitability of the Clinical Data Interchange Standards Consortium (CDISC) Operational Data Model (ODM) - ScienceDirect
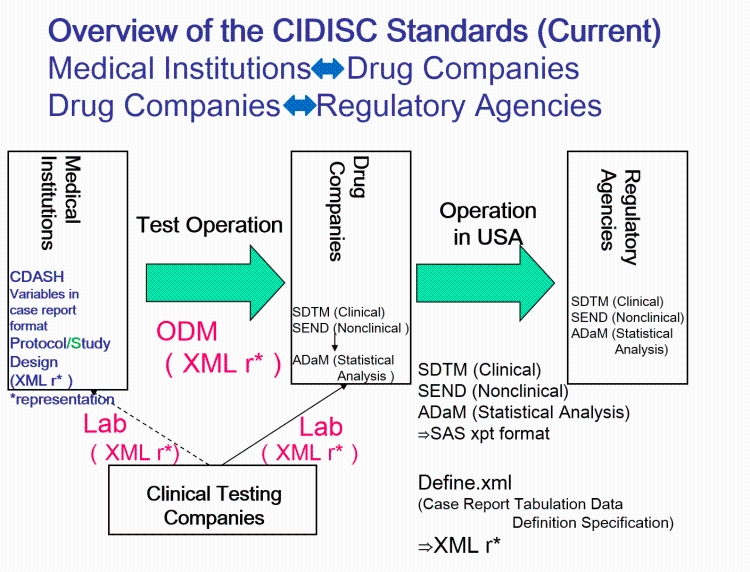
Joining the Clinical Data Interchange Standards Consortium (CDISC) and Activity Status | Japan Agency for Medical Research and Development

Benefits of Clinical Data Interchange Standards Consortium (CDISC) to Healthcare IT | Covetus Technologies Pvt Ltd
![PDF] CDISC Transformer: a metadata-based transformation tool for clinical trial and research data into CDISC standards | Semantic Scholar PDF] CDISC Transformer: a metadata-based transformation tool for clinical trial and research data into CDISC standards | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/39c5a0f62852e66837394ef1953006c33a1d8587/6-Figure3-1.png)
PDF] CDISC Transformer: a metadata-based transformation tool for clinical trial and research data into CDISC standards | Semantic Scholar

OpenClinica becomes Gold Member with the Clinical Data Interchange Standards Consortium (CDISC) » OpenClinica
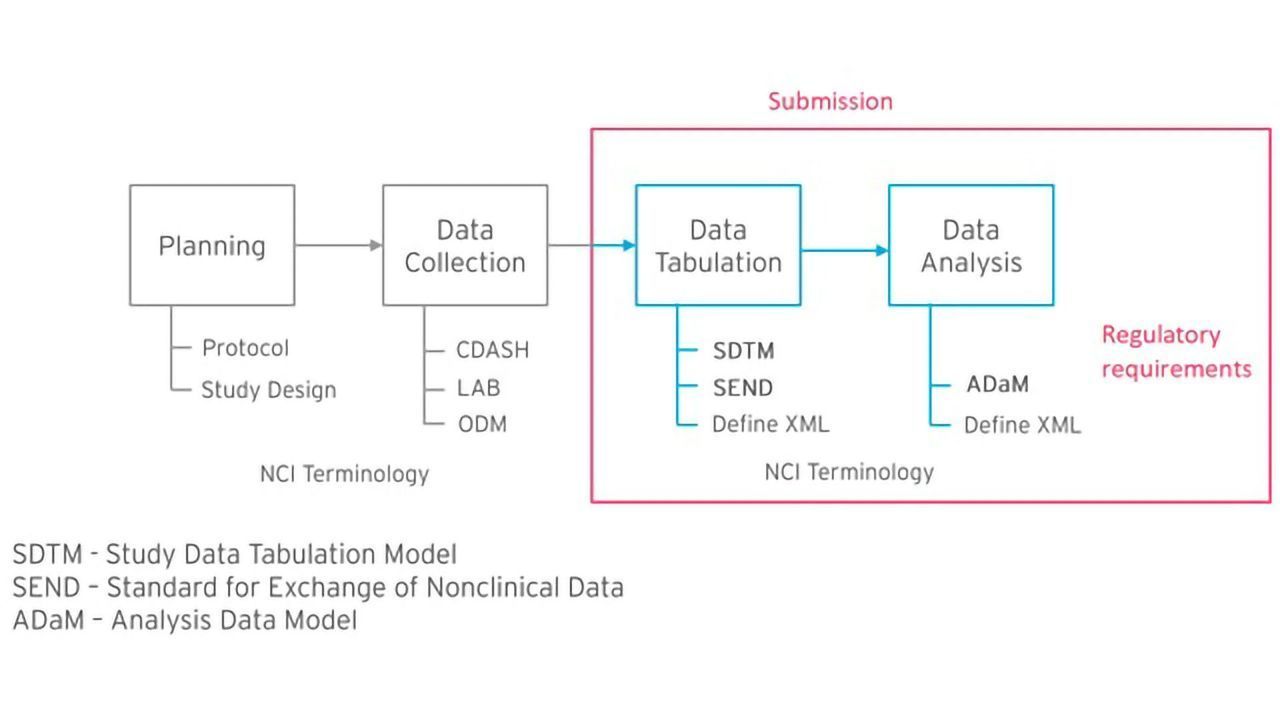
Formedix and CDISC Join Forces to Provide Free Access to Electronic Case Report Forms for Accelerated Clinical Trial Set-Up | Technology Networks

Understanding of data capturing in standardized CDISC-SDTM format – Clinical trials and CRO centric systems

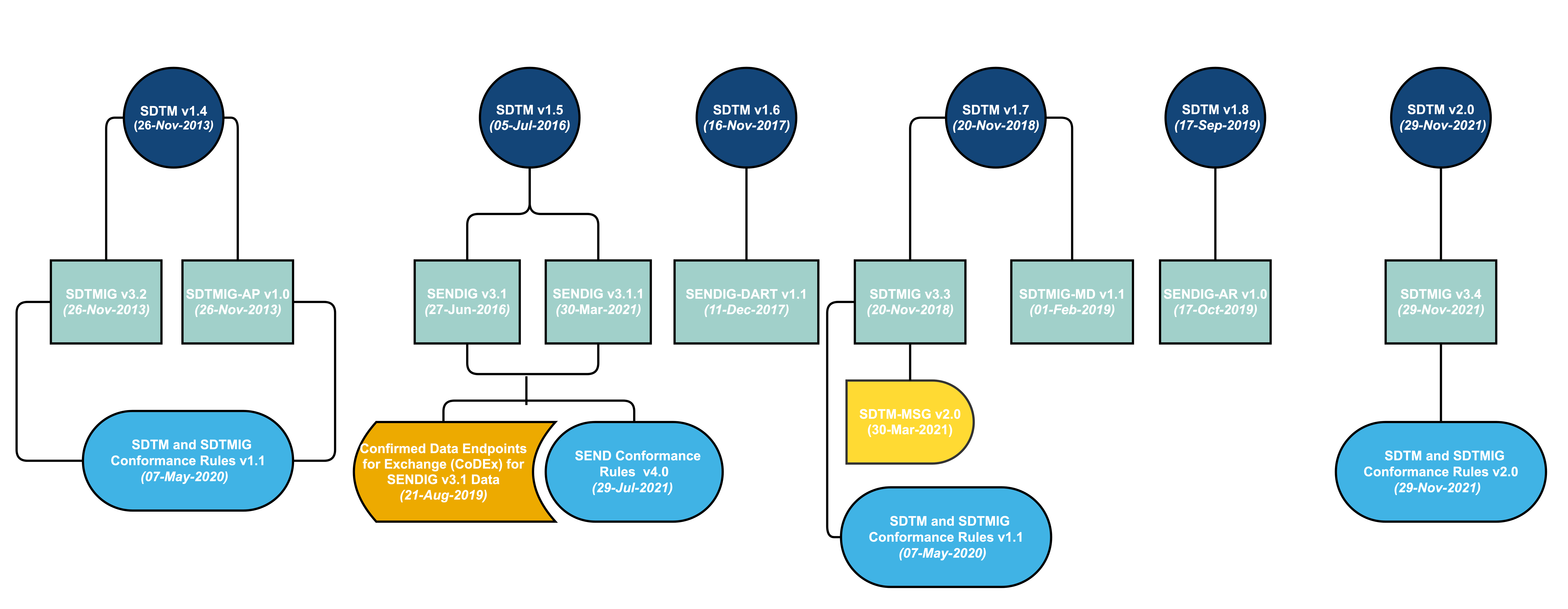






.png)